Get access now to LCAL2003: Chemistry labs (ultraconcurrent)
For only:
$9.99One payment
6 months of access
Payment methods supported:
- DigiKey (Not available in every country)
- Credit or debit card
- PayPal
You need to create or use a LabsLand account to continue.
Remote access to 12 laboratories included:
Trying to buy multiple licenses for a class? Contact us for bulk discounts
What is LabsLand?
LabsLand is the global network of remote laboratories.
The equipment is always real, not a simulation.
You control the real equipment with webcams through the Internet.
Access now. No need to wait for an equipment to be shipped.
No hidden costs: all included. No accessories or shipping costs.
Very easy to use: the equipment is already working.
Rent it only the months you need for your learning.
How does LabsLand work?
LabsLand is a global network of real laboratories available online. Students (in schools, universities and life-long learning platforms) can access the real laboratories through the Internet, using their laptop, tablet or phone.
The laboratories are either real-time (Arduino, FPGAs...) located in different multiple universities all over the world. In certain fields (Physics, Biology, Chemistry) the laboratories are LabsLand Ultraconcurrent Laboratories, so the university has recorded all the potential combinations of what can be done in the laboratory (in some cases, several thousands) and make it available in an interactive way.
In every case, the laboratory is always real (not simulated), and available through the Web (you do not need to obtain any hardware, deal with shipping, etc.).
Check how a typical user session works in the following video:
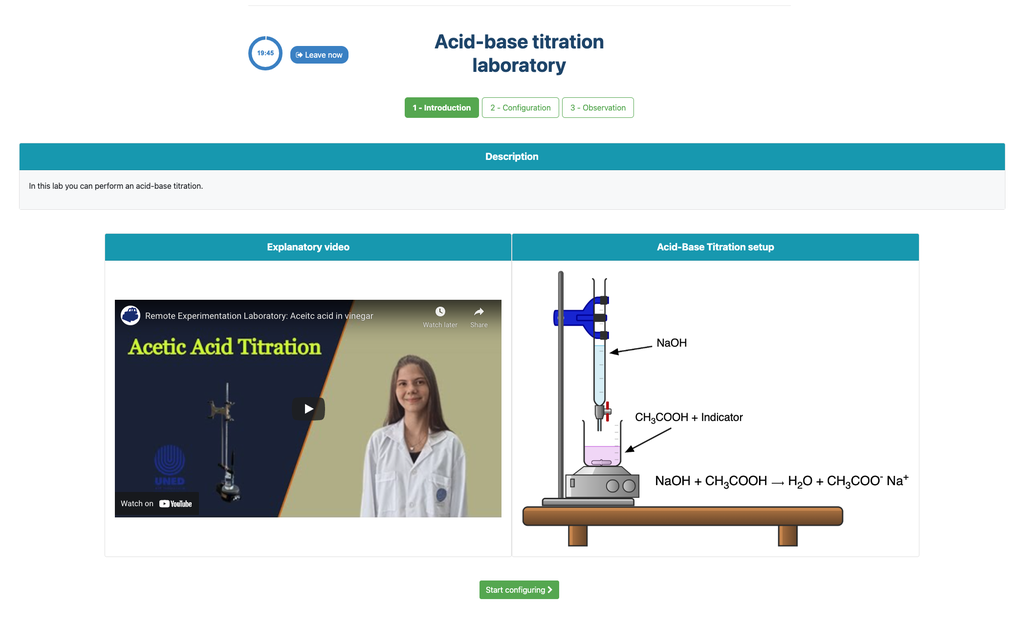
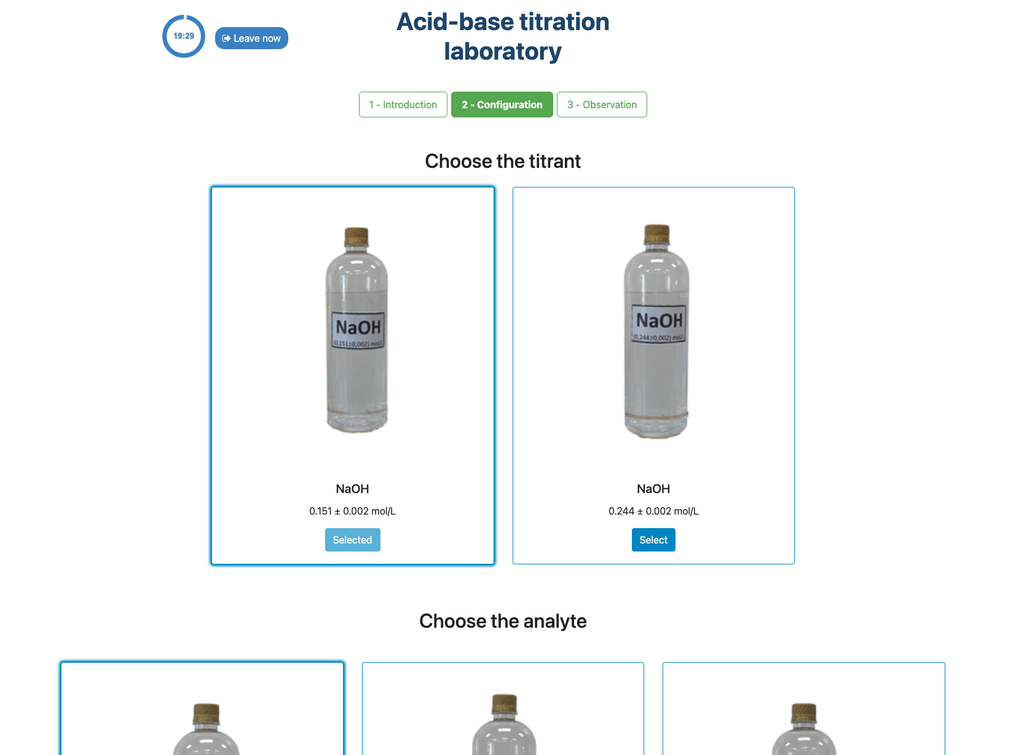
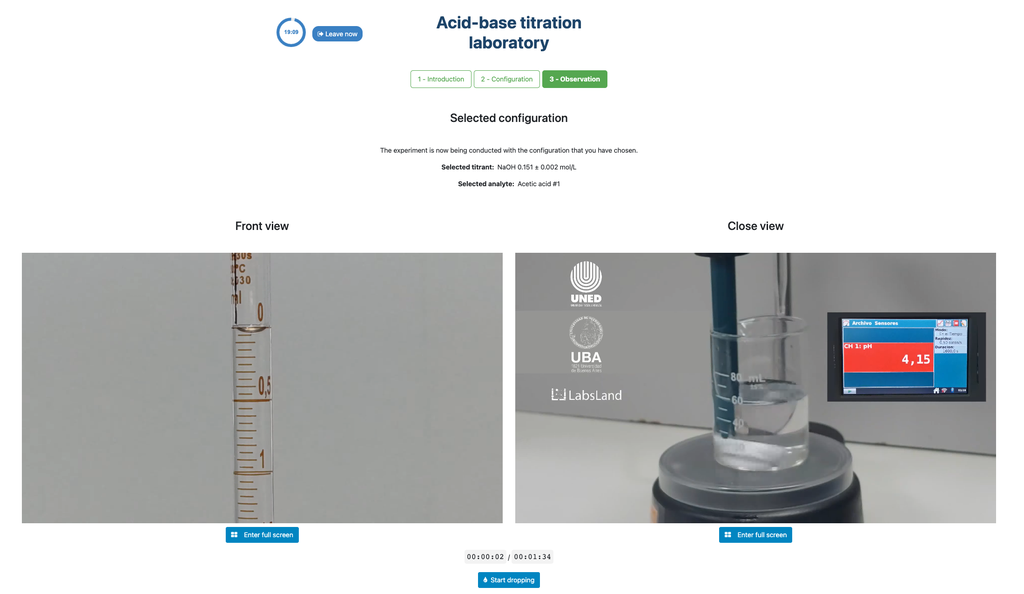
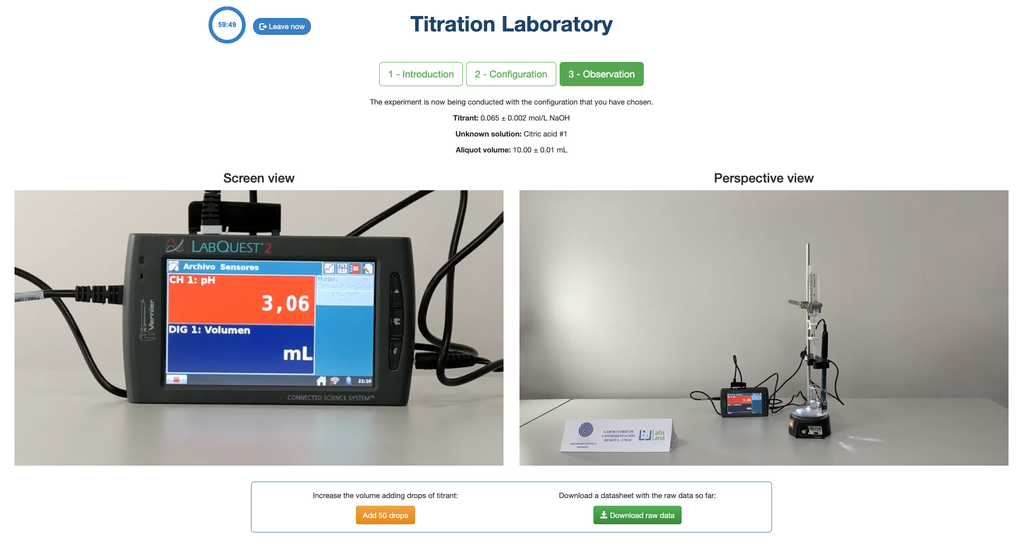
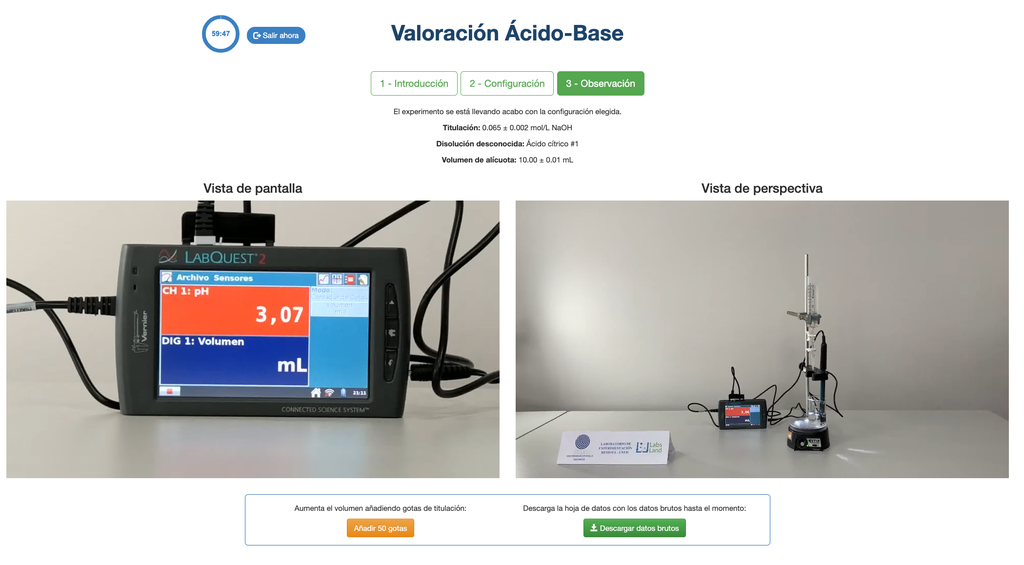
Acid Base Titration II
Summary
Perform an acid-base titration to determine the concentration of an unknown acetic acid solution using a sodium hydroxide titrant. This laboratory emphasizes visual measurements dealing with the meniscus of the burette, and supports two different configurations.
The first one is for a potentiometric approach: you will have access to a digital pH sensor and you can use it to determine when the unknown solution has been neutralized.
The second one is for a colorimetric approach: you can rely on the color change due to the presence of a phenolphthalein indicator, without having a digital pH sensor available.
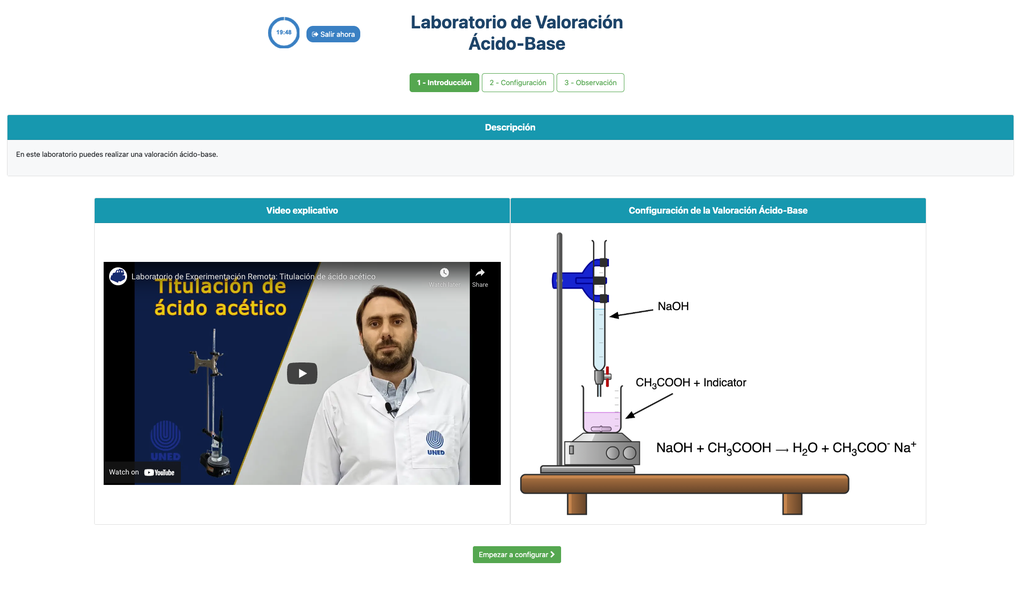
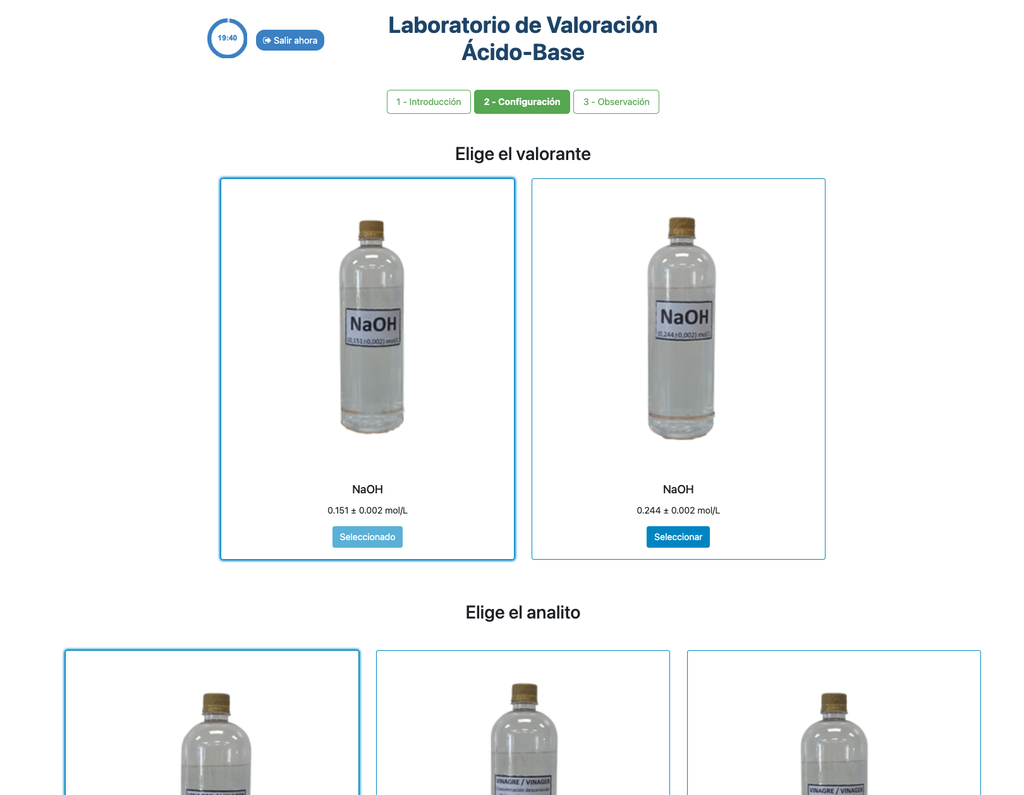
Acid-base Titration
Titrations are a volumetric method that is based on measuring the amount of a known-concentration reactive (known as a primary standard) that is consumed by an unknown-concentration sample known as analyte.
The titration is conducted by adding the titrant to the analyte using a burette, so as to obtain a chemically-equivalent substance between the titrant and the analyte. This is known as the "equivalence point" and it is a theoretical value that cannot be experimentally determined.
The experimental estimation of this point is obtained through an approximation known as "final point". This is determined through a physical change. In that case, the change in color of the solution is achieved after adding an indicator substance: a substance that changes color in certain ranges of pH.
For the acid-base titration we use a phenolphthalein indicator that becomes a light pink after a pH of around 8.4, which is a value that is very close to the equivalence point in the most common acid-base titrations.
Alternatively, in the potentiometric configuration, a digital pH sensor can be used to determine the “equivalence point”.
Colorimetric vs Potentiometric approach
The colorimetric approach relies on the color change provided by the phenolphthalein indicator. The potentiometric approach relies instead on the pH raise as measured by the digital sensor. In this version of the laboratory there are two different configurations available, one for each approach. In the colorimetric configuration students may not see the digital pH sensor.
Differences with the Acid-Base Titration II laboratory
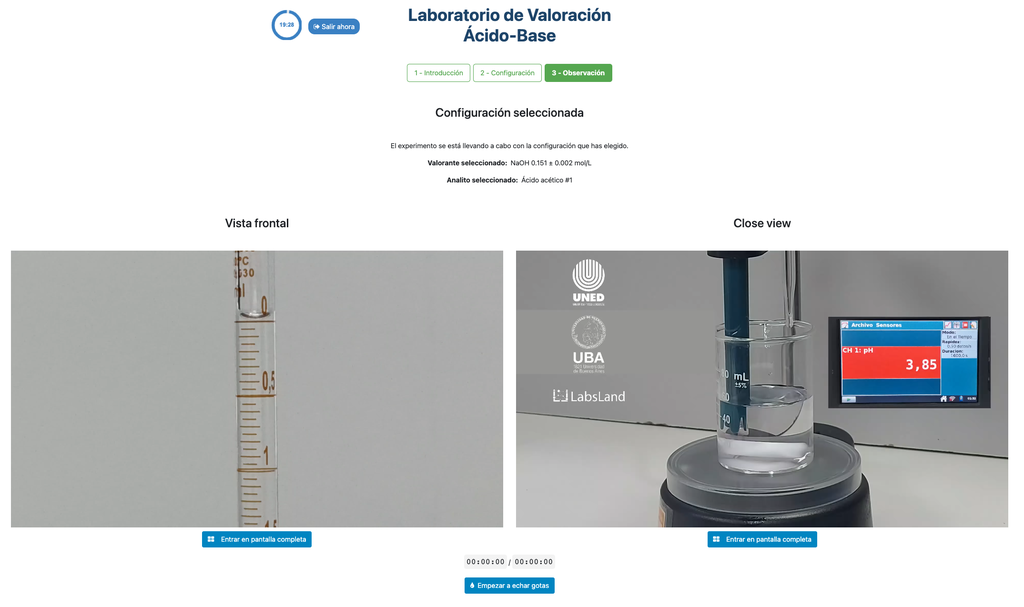
In this version of the laboratory (Acid-Base Titration II) you can perform the acid-base titration for an unknown acetic acid solution. In the other version of the laboratory (see Acid-Base Titration I) you can perform the acid-base titration for a citric acid solution instead.
This version of the laboratory emphasizes visual burette measurements, including properly reading the meniscus in the burette. The other version of the laboratory (see Acid-Base Titration I) does not emphasize this, and focuses on the calculations instead.
Also, in this version you can choose between two different configurations: one for the potentiometric approach and one for the colorimetric approach. The configuration for the colorimetric approach does not show the pH sensor. In the other version of the laboratory (see Acid-Base Titration I) there is a single configuration and the sensor is always shown.
Acid Base Titration III
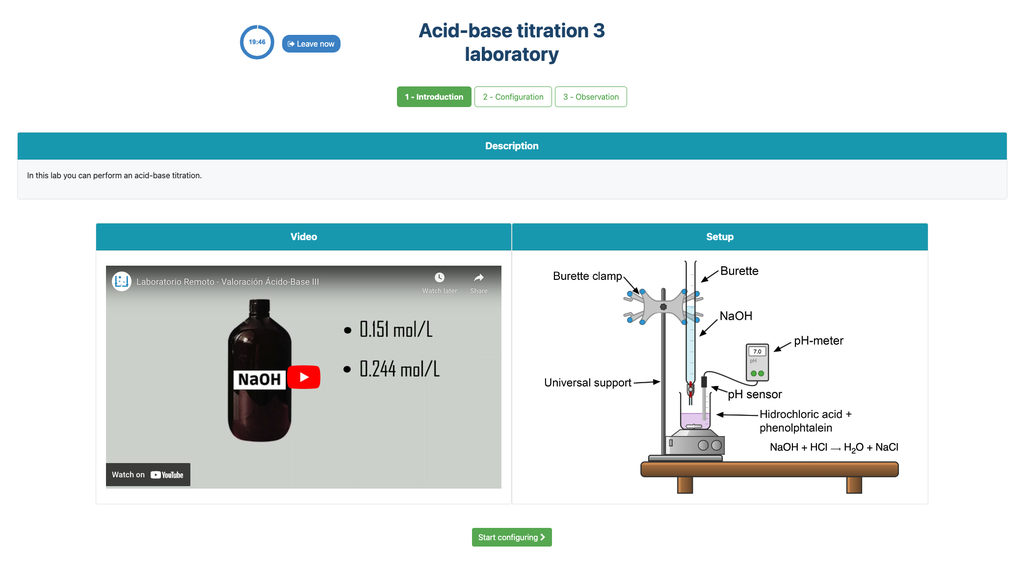
Summary
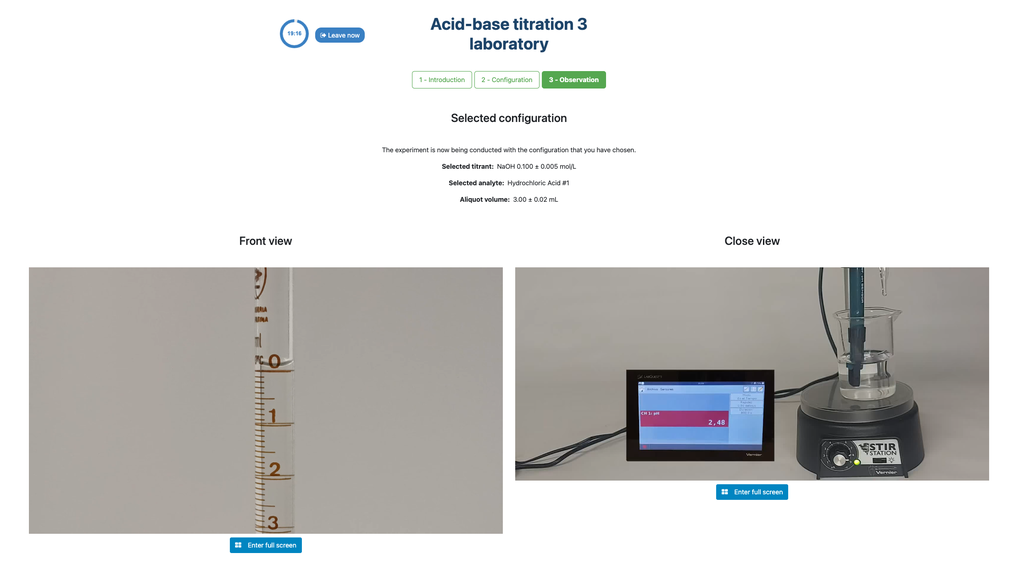
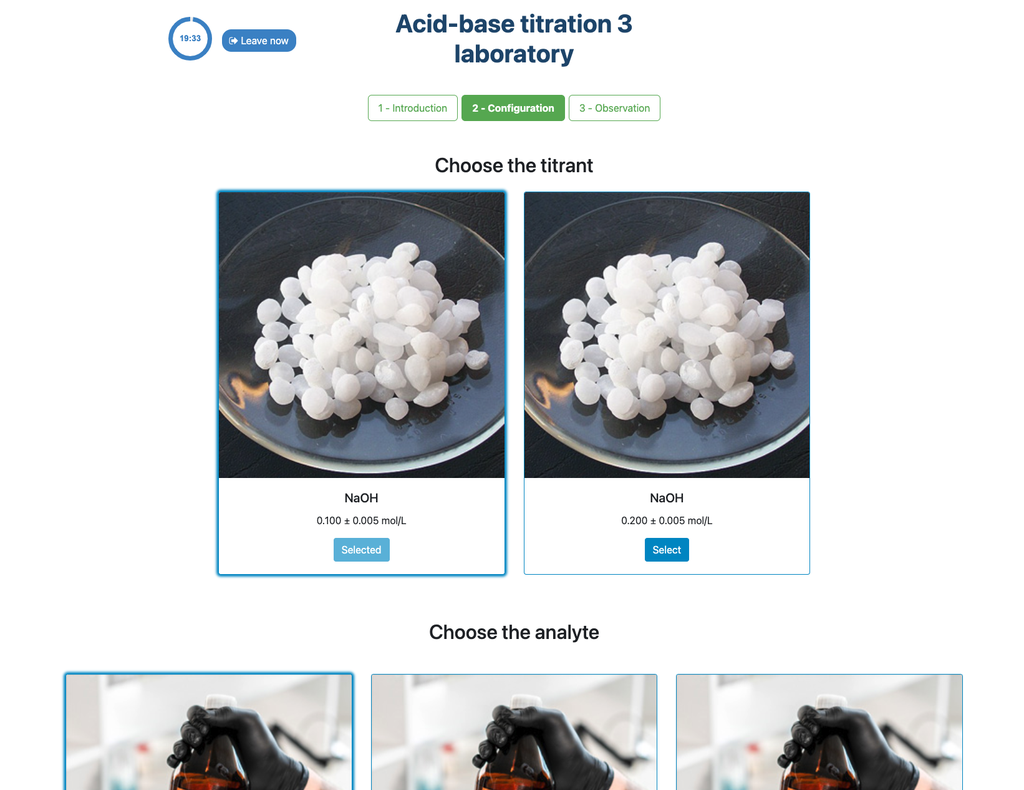
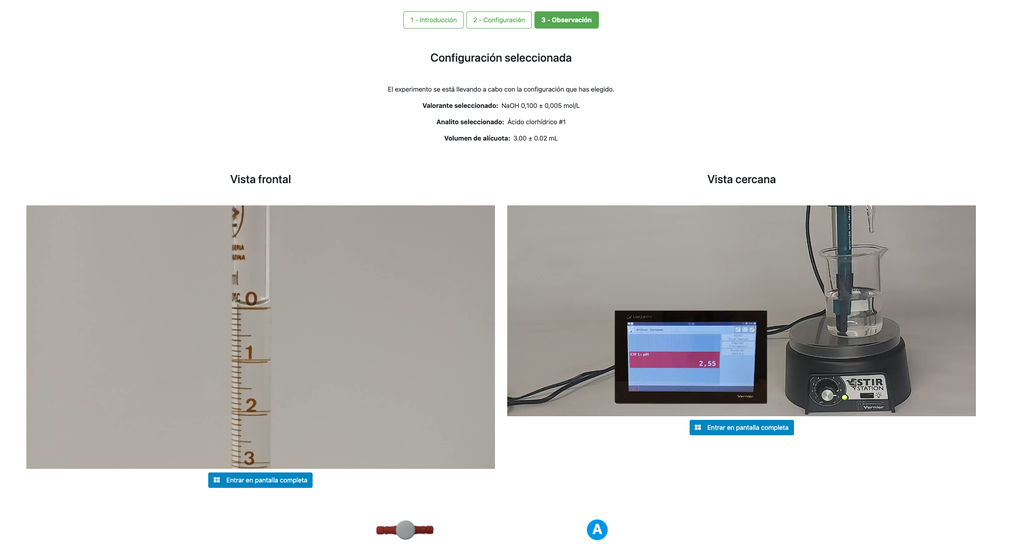
Perform an acid-base titration to determine the concentration of an unknown hydrochloric acid solution using a sodium hydroxide titrant. This laboratory emphasizes visual measurements dealing with the meniscus of the burette, and supports two different configurations.
The first one is for a potentiometric approach: you will have access to a digital pH sensor and you can use it to determine when the unknown solution has been neutralized.
The second one is for a colorimetric approach: you can rely on the color change due to the presence of a phenolphthalein indicator, without having a digital pH sensor available.
Acid-base Titration
Titrations are a volumetric method that is based on measuring the amount of a known-concentration reactive (known as a primary standard) that is consumed by an unknown-concentration sample known as analyte.
The titration is conducted by adding the titrant to the analyte using a burette, so as to obtain a chemically-equivalent substance between the titrant and the analyte. This is known as the "equivalence point" and it is a theoretical value that cannot be experimentally determined.
The experimental estimation of this point is obtained through an approximation known as "final point". This is determined through a physical change. In that case, the change in color of the solution is achieved after adding an indicator substance: a substance that changes color in certain ranges of pH.
For the acid-base titration we use a phenolphthalein indicator that becomes a light pink after a pH of around 8.4, which is a value that is very close to the equivalence point in the most common acid-base titrations.
Alternatively, in the potentiometric configuration, a digital pH sensor can be used to determine the “equivalence point”.
Colorimetric vs Potentiometric approach
The colorimetric approach relies on the color change provided by the phenolphthalein indicator. The potentiometric approach relies instead on the pH raise as measured by the digital sensor. In this version of the laboratory there are two different configurations available, one for each approach. In the colorimetric configuration students may not see the digital pH sensor.
Differences with the other titration laboratories
In this version of the laboratory (Acid-Base Titration II) you can perform the acid-base titration for an unknown hydrochloric acid solution. In other versions of the laboratory you can perform the acid-base titration for a citric acid solution instead (Acid-Base Titration I), and acetic acid (Acid-Base Titration II).
Both this version of the laboratory (Acid-Base Titration III) and Acid-Base Titration II emphasize visual burette measurements, including properly reading the meniscus in the burette. The other version of the laboratory (see Acid-Base Titration I) does not emphasize this, and focuses on the calculations instead.
Also, in these versions you can choose between two different configurations: one for the potentiometric approach and one for the colorimetric approach. The configuration for the colorimetric approach does not show the pH sensor. In other version of the laboratory (see Acid-Base Titration I) there is a single configuration and the sensor is always shown.
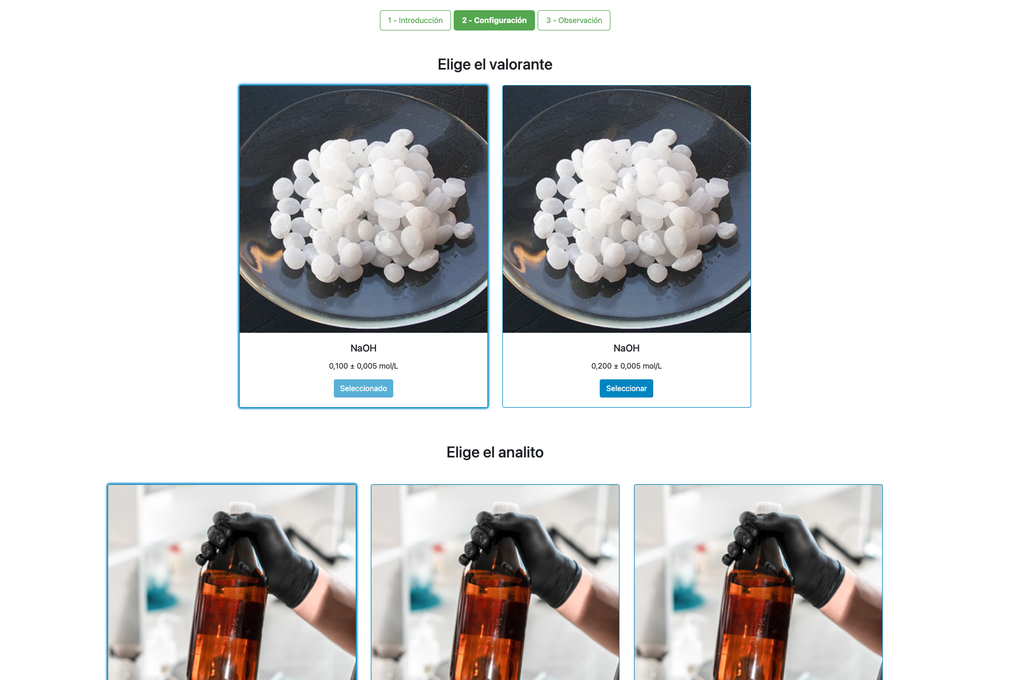

Acid-Base Titration I
Summary
Perform an acid-base titration to determine the concentration of an unknown citric acid solution using a sodium hydroxide titrant. A digital pH sensor is always available and a phenolphthalein indicator has been applied to the unknown solution so that both a potentiometric and colorimetric approach can be used. A real-time plot is also available.
Acid-Base Titration
Titrations are a volumetric method that is based on measuring the amount of a known-concentration reactive (known as a primary standard) that is consumed by an unknown-concentration sample known as analyte.
The titration is conducted by adding the titrant to the analyte using a burette, so as to obtain a chemically-equivalent substance between the titrant and the analyte. This is known as the "equivalence point" and it is a theoretical value that cannot be experimentally determined.
The experimental estimation of this point is obtained through an approximation known as "final point". This is determined through a physical change. In that case, the change in color of the solution is achieved after adding an indicator substance: a substance that changes color in certain ranges of pH.
For the acid-base titration we use a phenolphthalein indicator that becomes a light pink after a pH of around 8.4, which is a value that is very close to the equivalence point in the most common acid-base titrations.
Colorimetric vs Potentiometric approaches
The colorimetric approach relies on the color change provided by the phenolphthalein indicator. The potentiometric approach relies instead on the pH raise as measured by the digital sensor. In this version of the acid-base titration laboratory both approaches can be used. The digital sensor is always available and cannot be hidden.
Differences with the Acid-Base Titration II laboratory
In this version of the laboratory (Acid-Base Titration I) you can perform the acid-base titration for an unknown citric acid solution. In the other version of the laboratory (see Acid-Base Titration II) you can perform the acid-base titration for an acetic acid solution instead.
This version of the laboratory emphasizes the calculations but does not have visual burette measurements among its learning objectives. The other version of the laboratory (see Acid-Base Titration II) emphasizes visual measurements, and students must learn to read the meniscus of the burette properly.
Also, in this version there is a single experience that can be used for both the colorimetric and potentiometric approaches. In the other version of the laboratory (see Acid-Base Titration II) there are two different configurations available, in one of which the digital sensor is hidden so that students may only rely on the color change.
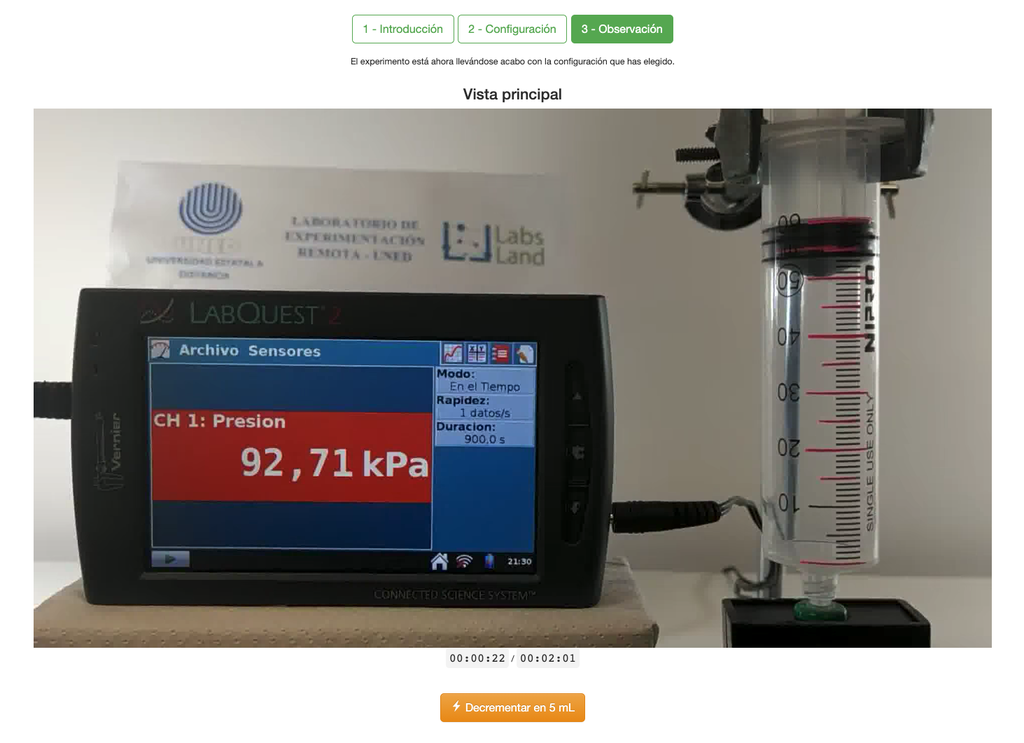
Boyle's Law
Summary
The Boyle's Law laboratory allows students to determine the relationship between the pressure and volume of a gas at ambient and constant temperature. Students can choose from two different volume syringes and measure the pressure of the gas as they reduce the volume. The experiment is reflected in a graphical analysis in the form of an isotherm. In this way, they can verify Boyle's Law and learn about the behavior of gases in a practical and accessible manner.
Boyle's Law
Boyle's Law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. This means that when the pressure of a gas increases, its volume decreases, and vice versa. Boyle's Law can be mathematically expressed as:
V ∝ 1/P
Where V is the volume of the gas and P is the pressure of the gas.
The Boyle's Law laboratory allows students to put this law into practice and verify it in an experimental context. By measuring the volume and pressure of the gas at different times, they can plot an isotherm graph that shows how the volume of the gas changes based on its pressure. If the isotherm graph fits Boyle's Law, then the students have experimentally verified the law.
Performing experiments like this is an excellent way to learn about the behavior of gases and how different variables are related. In addition, hands-on experiments can be more accessible and memorable for students than simply reading about the law in a textbook. The isotherm graph clearly visualizes the behavior of the gas and verifies if the predictions of Boyle's Law are met.
Application in Secondary and University Education
The Boyle's Law laboratory is typically applied in science courses at the high school level and in chemistry courses at the university level. At the high school level, the laboratory can be applied in a science course where the basic concepts of chemistry and physics, such as the pressure and volume of gases, are studied. At the university, the Boyle's Law laboratory can be applied in a more advanced chemistry course where the study of gases and their behavior is delved into.
Objectives
A Boyle's Law laboratory can have different educational objectives depending on the educational level at which it is applied. Here are some examples of objectives that a Boyle's Law laboratory can have at both high school and university levels:
At the high school level:
- Students understand Boyle's Law and its importance in gas physics.
- Students practice experimental and observational skills.
- Students develop data analysis and representation skills.
- Students understand the relationship between temperature, pressure, and volume of gases.
At the university level:
- Students know Boyle's Law and its importance in gas physics.
- Students demonstrate experimental and observational skills in the laboratory.
- Students apply theoretical concepts of gas physics in practical situations.
- Students develop data analysis and representation skills in a scientific context.
- Students understand how temperature, pressure, and volume of gases relate in different situations.
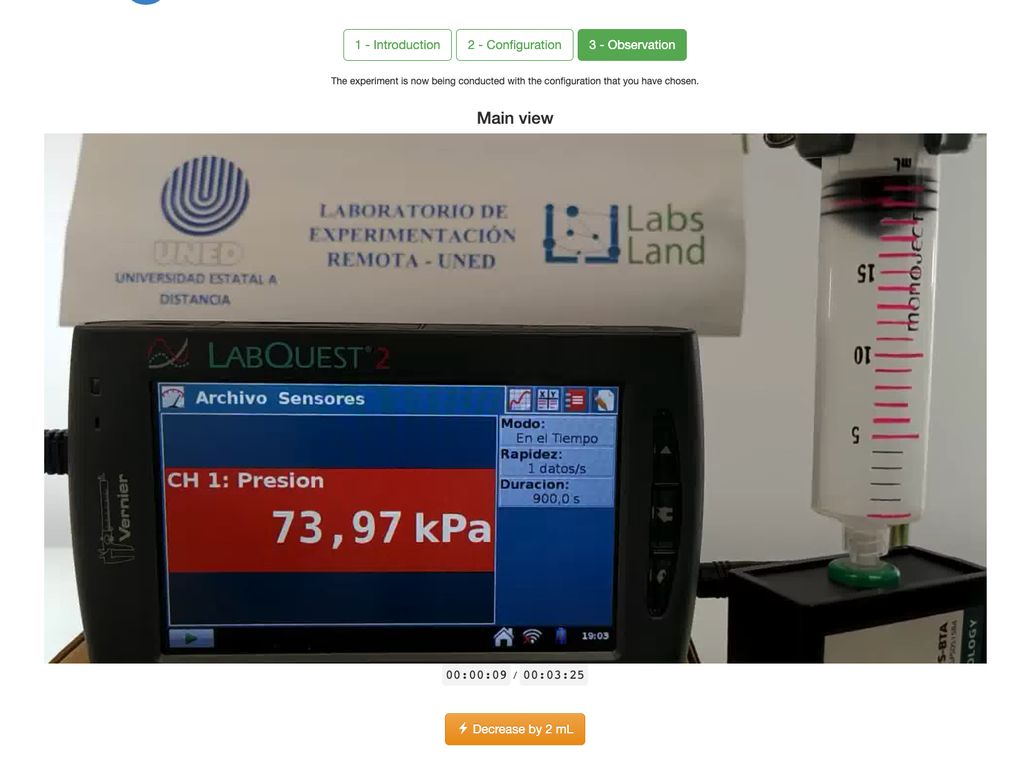
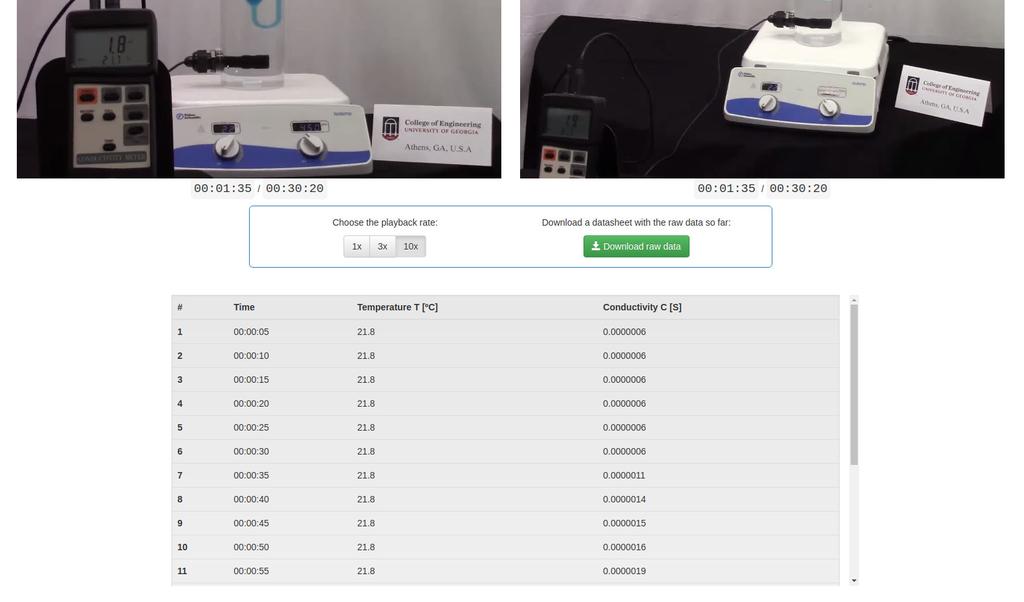
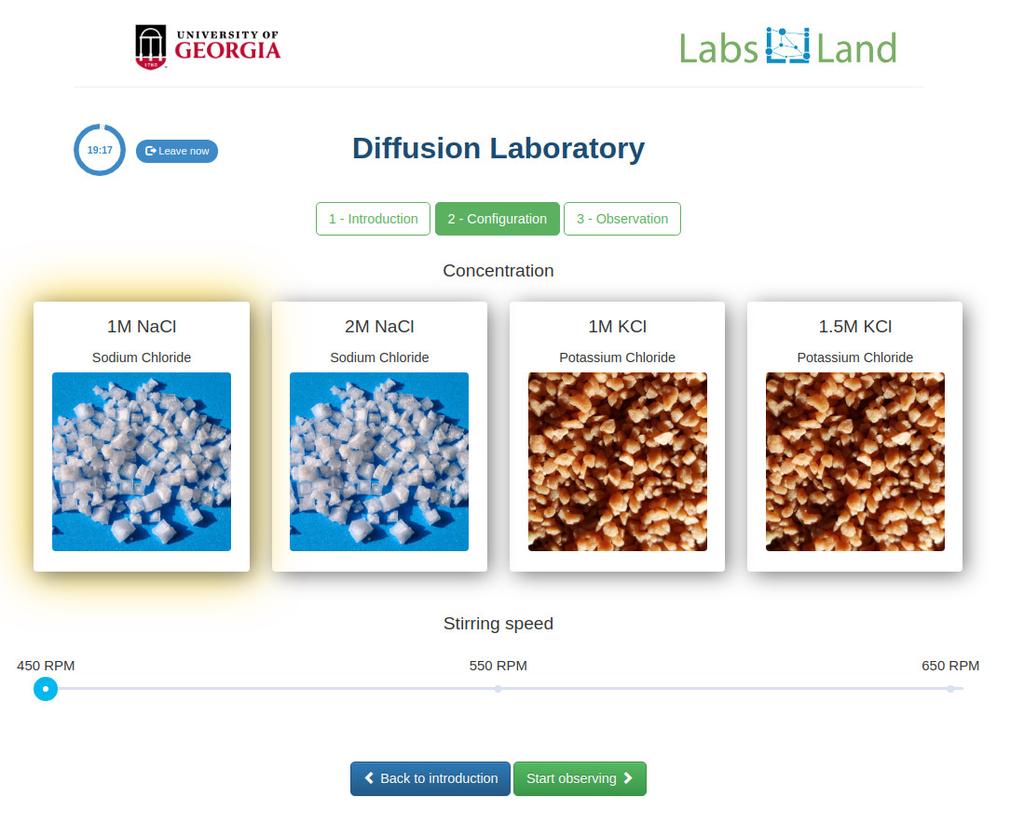
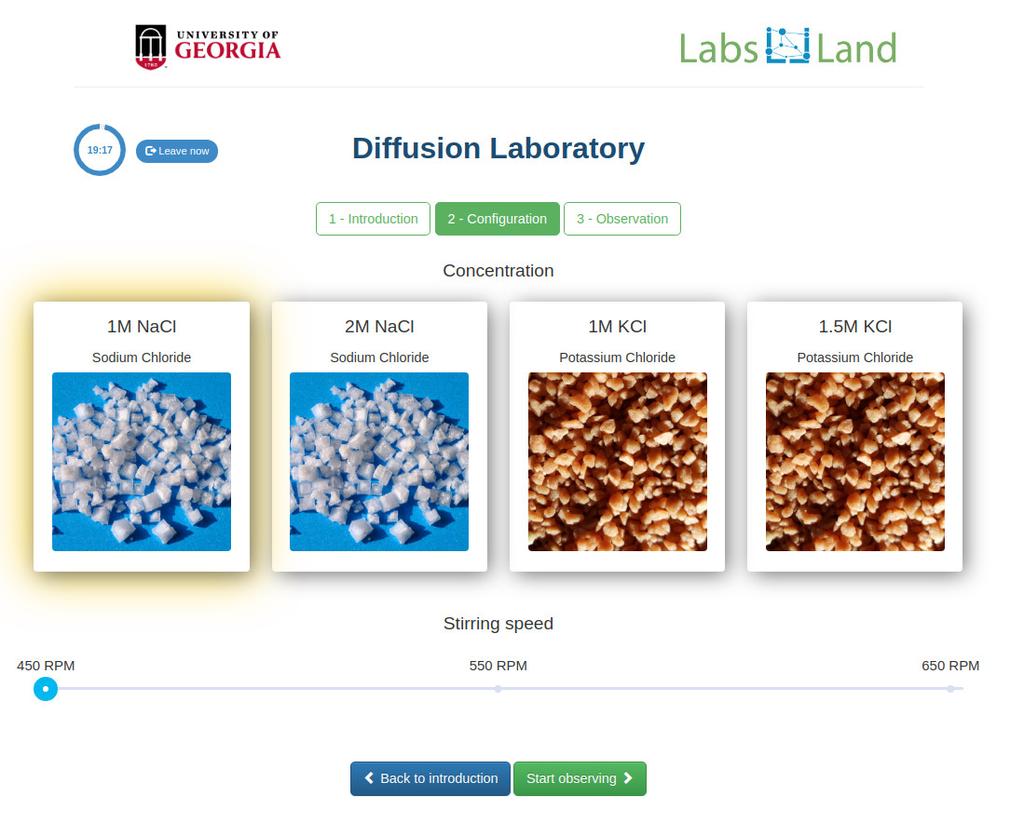
Diffusion laboratory - basic
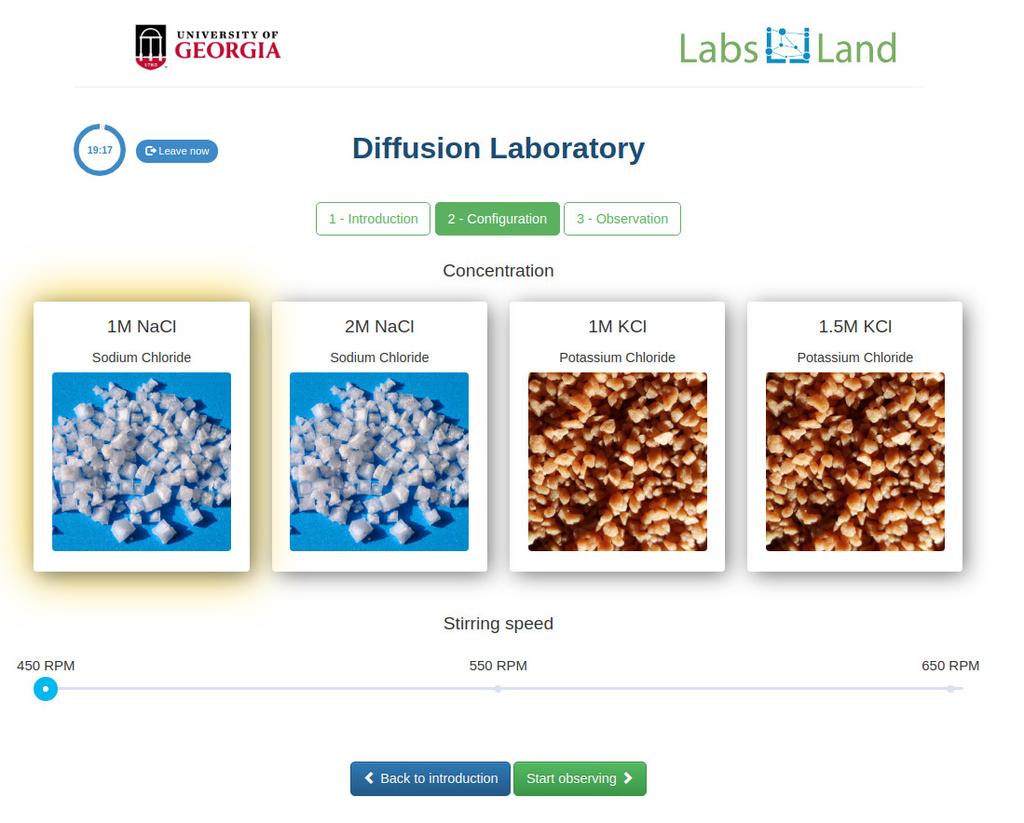
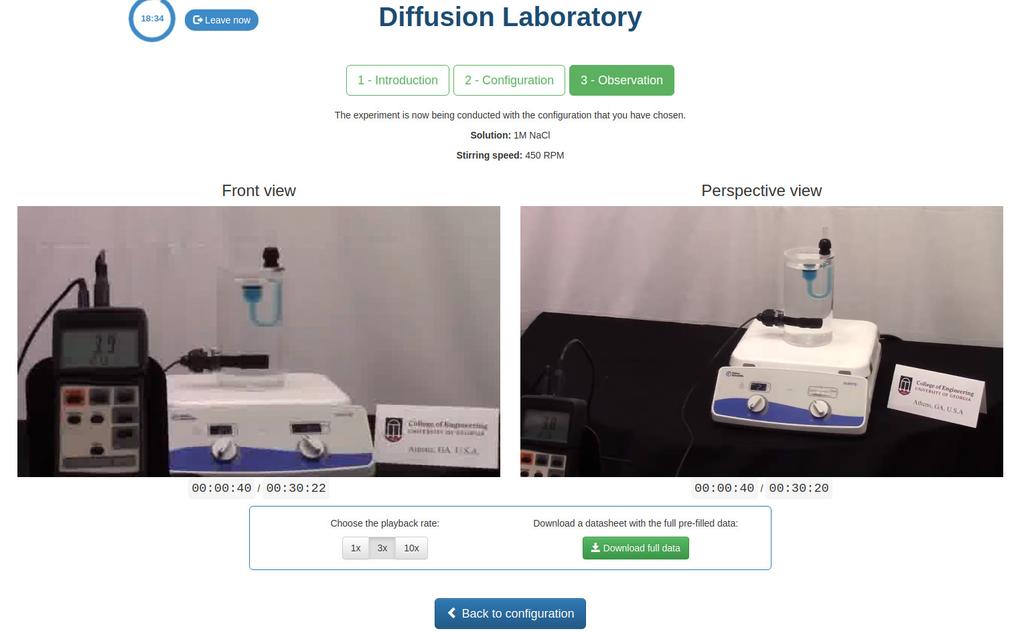
Diffusion laboratory - data
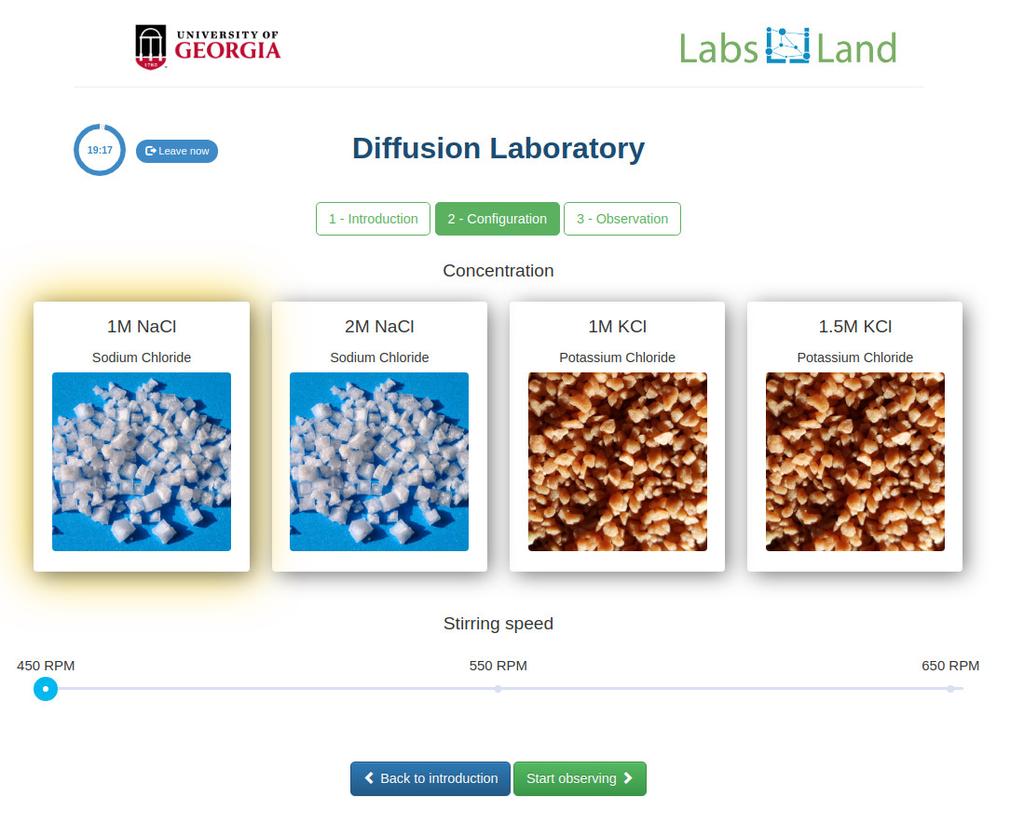
Diffusion laboratory - full
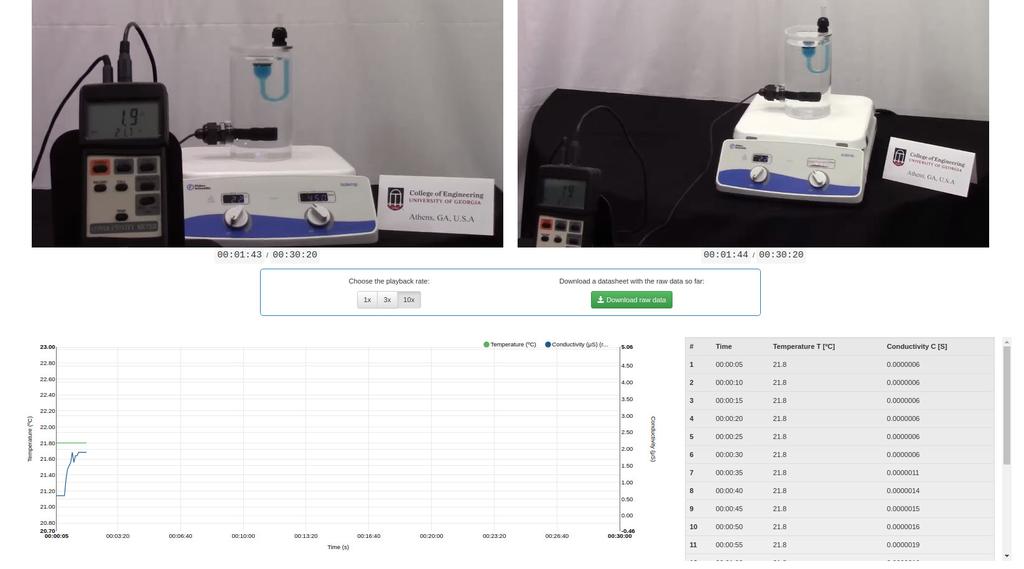
Diffusion laboratory - plot
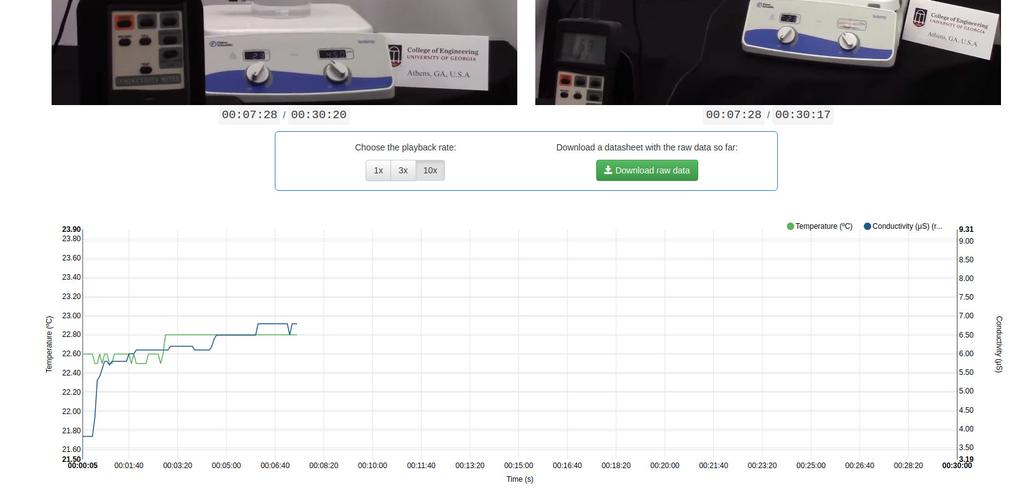
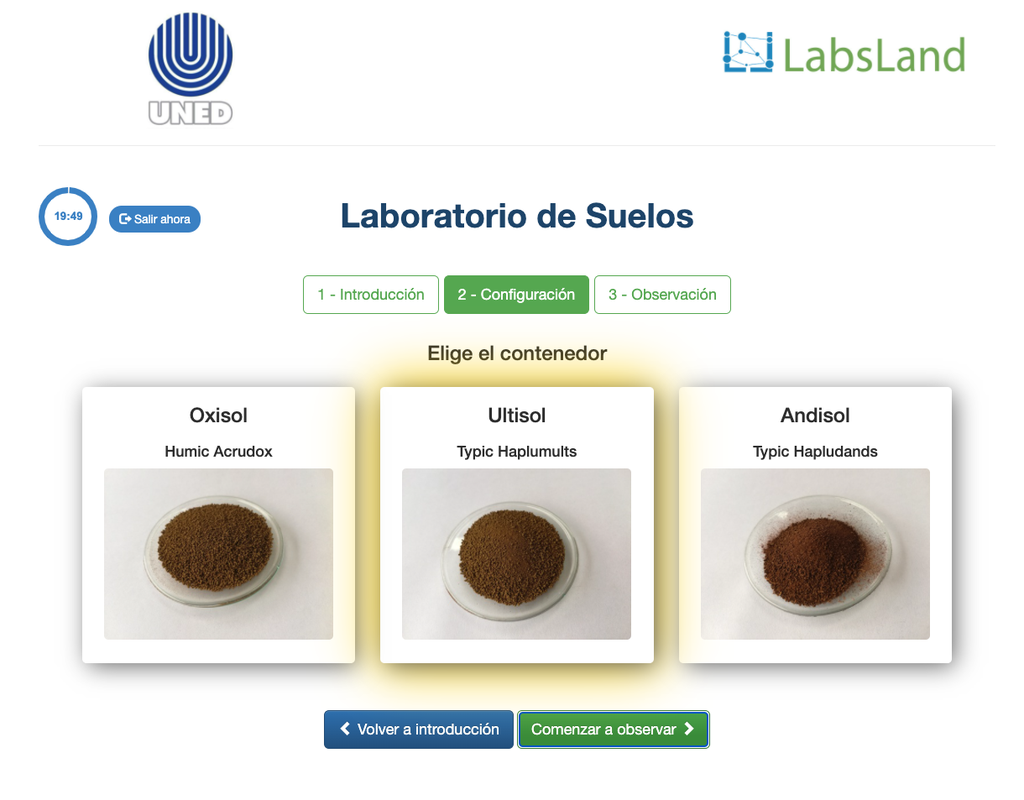
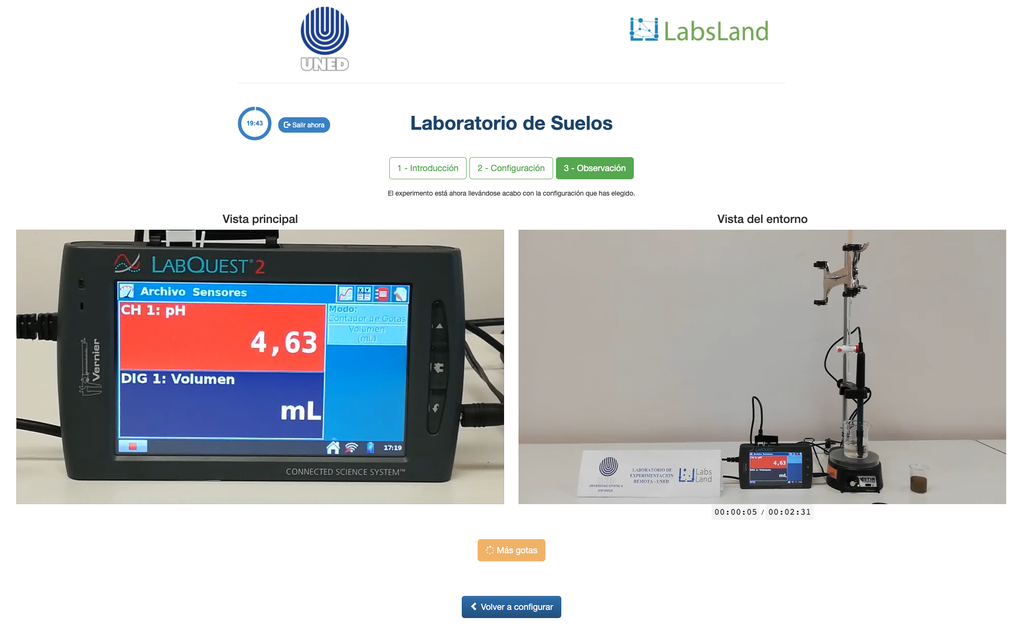
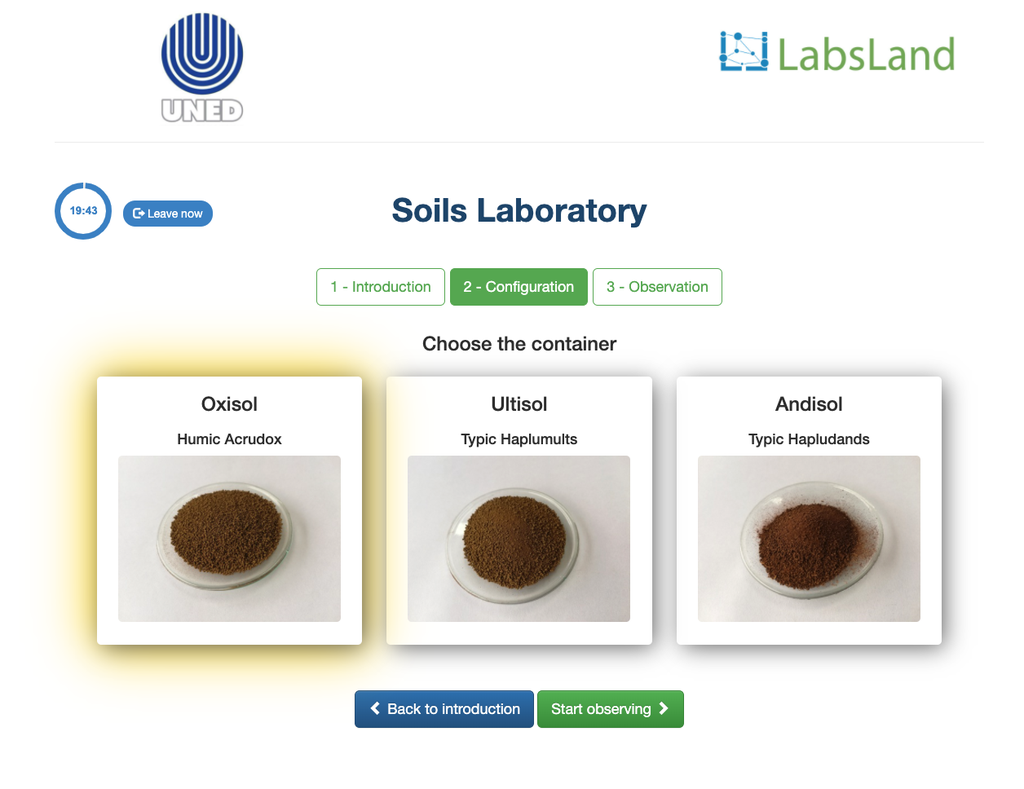
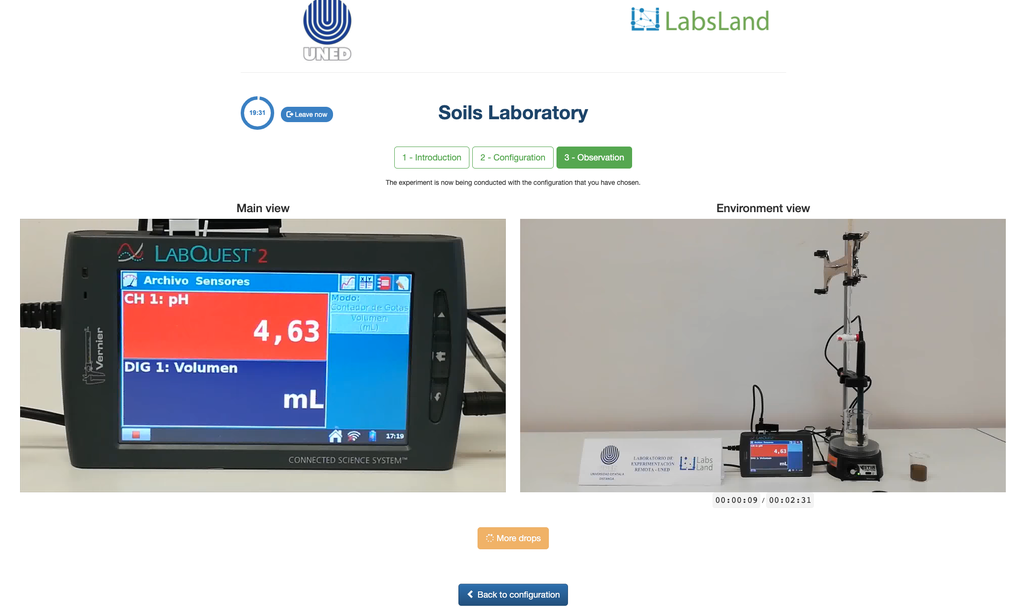
Exchangeable Acidity of Soils
The acidity of soils can occur due to various processes that promote a pH reduction. These processes occur naturally or by human action. The main sources of soil acidity are associated to hydrogen ions (H+) and aluminum ions (Al+3) in the soil's solution. Exchangeable acidity is determined through the use of neutral salts solutions such as potassium chloride (KCl). The acid ions (aluminum and hydronium) that are held in the colloidal fraction of the soil, that in the presence of a displacing ion (K+), makes those enter the soil solution. Afterward, that solution is titrated with a sodium hydroxide solution of the exact concentration to reach the last point of the neutralization reaction using phenolphthalein as an indicator.
Gay-Lussac's Law
Gay-Lussac's law allows us to study the behavior of gases and is often studied in physics and chemistry. It relates the pressure of a gas with its temperature, while other parameters such as volume and amount remain constant.
There are various way to verify Gay-Lussac's law. In this experiment we will verify that, for a given amount of the gas, the pressure is directly proportional to the temperature.
Radioactivity
Summary
The setup, useful for schools and universities, includes a Geiger counter that can measure the number of detected particle collisions. The user can choose among different radioactive sources, as well as an absorber to put between the radioactive source and the probe. Additionally, other parameters that users can vary are the distance and number of tests. This allows for a wide range of experiments and learning opportunities.
Radioactivity
Radioactivity is the process in which an atomic nucleus loses energy by emitting particles and radiation. This can occur naturally in certain elements, or artificially through the use of nuclear reactions. In the context of physics at schools and universities, studying radioactivity can provide valuable insights into the fundamental nature of matter and the laws of physics.
One common experiment in this area is the measurement of radioactivity using a Geiger counter. This instrument is able to detect the emission of particles from a radioactive source, allowing students to understand the basic principles of radiation and its effects on matter. By varying the type of radioactive source, the distance between the source and the detector, and the type of absorber material placed between the two, students can explore a wide range of phenomena and gain a deeper understanding of the underlying principles.
In addition to its educational value, studying radioactivity also has practical applications in fields such as medicine, energy production, and environmental protection. As such, it is an important topic for students to learn about, both for its intrinsic interest and for the many real-world applications it has.
Real-world applications of radioactivity
One of the most common applications of radioactivity is in the field of medicine. Radioactive isotopes are used in medical imaging techniques such as PET and SPECT scans, which allow doctors to see inside the body and diagnose diseases. Radioactive isotopes are also used in cancer treatments, such as radiotherapy, where they are used to kill cancer cells.
Radioactivity is also used in industries such as oil and gas exploration, where it is used to measure the permeability of rock formations and the flow of fluids through them. Radioactive isotopes are also used in smoke detectors and in the production of luminous watches and instruments.
Overall, radioactivity has a wide range of applications in fields such as medicine, industry, and even everyday consumer products. It continues to be an important area of study in physics and other sciences, and its uses continue to expand as new technologies are developed.
Radioactivity experiments at schools and universities
The use of a geiger counter in a radioactivity experiment allows for a wide range of possibilities. By varying the radiation emitters and absorbers, students can observe the effects of different sources and materials on the detected particle collisions. This can help students understand the properties of radioactivity and the behavior of different particles.
Additionally, experiments involving the determination of the type of particle being radiated can be conducted by observing whether the particle is absorbed or not. By placing different absorbers between the source and the probe, students can determine the properties of the emitted particles and gain a deeper understanding of radioactivity.
Lastly, experiments involving the determination of the geometric form of radioactivity emission can also be conducted using a geiger counter. By carefully measuring the detected particle collisions at different distances, students can gain insights into the spatial distribution of radioactivity. This can help students understand the fundamental principles of radioactivity and its applications in the real world.
Potential learning objectives
Potential objectives of activities conducted with the laboratory are the following:
- Understand the properties and behavior of radioactive emissions.
- Conduct experiments to measure the effects of radiation on various materials.
- Determine the type of radiation emitted by a radioactive source.
- Understand the principles of radiation safety and handling.
- Investigate the applications of radioactivity in fields such as medicine, industry, and research.
- Understand the principles of Geiger counters and their use in measuring radioactivity.
- Understand the historical development of the concept of radioactivity and its discovery.
- Explore the ethical implications of the use of radioactive materials.

Spectroscopy
This ultraconcurrent laboratory is based in an experimental practice about X-ray spectroscopy using a LEYBOLD-brand device that is installed in a radiological instrumentation laboratory in the National University of Costa Rica (Universidad Nacional de Costa Rica), located in the Applied Medical Physics building.
The assembly consists of an X-ray tube with a gold (Au) anode, together with a scintillation detector configured with a preamplifier and a digitizer that allows processing information from the measurements made by the detector through software.
The test aims to characterize the beam of the radiation source through the experimental calculation of the spectrum of the X-ray beam produced in the tube, in addition to generating basic notions about radiological instrumentation and how the variation of its parameters is used in industrial and medical applications.