We have developed a new remote chemistry laboratory, together with the Universidad Estatal a Distancia (UNED) in Costa Rica: the Titration laboratory. After having been used with great success in a test phase by several institutions and hundreds of students, the lab is now officially ready! It can already be found as a lab in our catalogue on the main portal, and those institutions with a LabsLand space and with plans that include chemistry can already add it to their groups and use it with their students. This lab is useful for schools and universities.
The UNED
The Universidad Estatal a Distancia (UNED) of Costa Rica is considered the first public university offering distance education in Costa Rica and Latin America and was founded in 1977. Today, the UNED offers a variety of technological means of distance learning, through different platforms.
Titration
Titration is a basic technique in chemistry, which aims to determine the concentration in a solution of unknown concentration. The unknown solution is usually called the “analyte” and is the unknown. In order to determine its concentration, this technique uses a solution of known concentration, usually known as a ‘titrant’. If the unknown solution is a base, an acid titrant is used. The titrant is poured over the analyte until it is neutralised. The point at which it is neutralised is known as the “equivalence point”. The researcher measures the precise volume of titrant that you need to add to the analyte. Since we know the concentration of the titrant, with this information we can subsequently determine the concentration of the analyte.
When conducting this technique, it is important to add the precise amount of titrant needed to reach the equivalence point. If additional titrant is added, the volume measured will not match the required volume, and the result of the calculation will therefore be incorrect.
For this reason, in general, it should be added gradually. To know when we have reached this point, we will rely on the fact that when we reach this point, there will be a drastic variation in the pH of the solution. We can use a pH sensor to detect this drastic variation. Alternatively, we can also use phenolphthalein to ensure that there is a visual change in the colour of the solution when a certain pH is reached. In the remote laboratory, both techniques can be applied: a pH sensor is present at all times, but a few drops of phenolphthalein have also been applied to the solution. Titration is a technique that is often taught both at secondary school level and in introductory chemistry courses at university level. LabsLand also has other remote chemistry-oriented laboratories, including the Diffusion and Boyle’s Law laboratories.
LabsLand’s remote laboratory
The Titration lab of LabsLand allows students to perform the above mentioned technique, including performing the related calculations, in a similar way as they would in a traditional on-site lab.
The student, upon entering the lab, will be able to choose between different titrants and different analytes. The student will then be shown the laboratory instruments, already prepared. In a classroom laboratory, they would have to be prepared beforehand. In this case, the teaching objective is not so much the manual part, but the application of the technique itself and the performance of the necessary calculations.
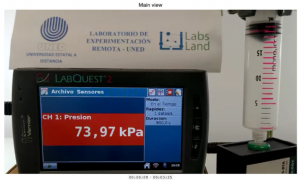
In the observation stage of the laboratory, we will mainly observe two views. Firstly, on the left, we will see the screen showing us the data from a real time pH sensor, together with the volume of analyte poured up to that moment. On the right, we will see a more distant view of the equipment. We will see, in particular, the burette containing the titrant, and the beaker containing the analyte.
The student can start pouring analyte into the titrant by pressing a button. The mentioned number of drops will then be poured, and the process and the resulting pH change can be observed. After the pH has stabilised, the pouring of drops can continue. The student himself or herself must realise at what point to stop pouring drops, so that the results of the calculations are correct. To determine this, the student can observe the graph generated with the data, wait for the sudden variation in pH, or observe a change in the colour of the solution to pink, due to the phenolphthalein indicator that has been applied to it.
Both views are shown in the image below.
Other laboratories with UNED
LabsLand has developed many other laboratories together with UNED, among them the Boyle’s Law Laboratory, the Gay-Lussac’s Law Laboratory and the Texturometer Laboratory. The first two are, like this lab, chemistry-oriented, and applicable at both school and university level. The Texturometer, also known as Texture Analyser, belongs to a different field.
The Remote Experimentation Laboratory of the UNED also has physical copies of various laboratories, including the Arduino Robot, the Arduino Basic and the VISIR (Electronics).