The Boyle’s Law chemistry lab, developed together with the Universidad Estatal a Distancia (UNED) of Costa Rica, is now available after a successful testing phase. This laboratory allows us to experimentally verify Boyle’s Law and perform related calculations. Boyle’s Law is one of the basic laws governing the behavior of gases, and is typically studied in introductory chemistry and physics courses.
The UNED of Costa Rica
The UNED of Costa Rica is the main distance learning university in this country. This distance learning university was founded in 1977, being the first university in both Latin America and Costa Rica with this modality. The UNED’s Remote Experimentation Laboratory has collaborated and continues to collaborate in the design and development of various laboratories.
Boyle’s Law
Boyle’s Law, also known as Boyle-Mariotte’s Law, is one of the basic laws explaining the behavior of gases. Specifically, Boyle’s Law relates the volume and pressure of a gas, assuming it is maintained at a constant temperature.
It is one of the gas laws typically taught in chemistry courses at both secondary school and university levels.
Adapted to the remote laboratory
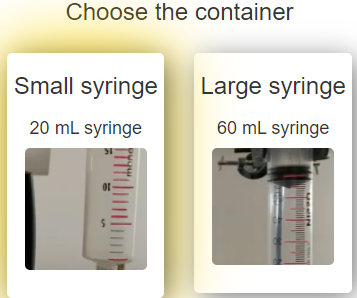
To conduct this experiment, we can use a small syringe (20 mL) or a large syringe (60 mL) as a container. Once you have chosen the type of syringe through the laboratory configuration screen, you can start the experiment. In the next observation stage, we will see the syringe that we have selected. A pressure sensor will have been attached to it, so that we can visually observe both the volume of the syringe and the pressure inside it at all times.
In the observation stage, moreover, students will not limit themselves to observing. In order to conduct the experiment they will generally have to virtually press the syringe in such a way that the pressure increases. To do this, they must press the “Decrease mL” option. In case of using the small syringe it will be possible to decrease by 2 mL each time and in the case of using the large syringe up to 5 mL each time.
In the image below you can see the different instruments (syringes) available to configure the laboratory.
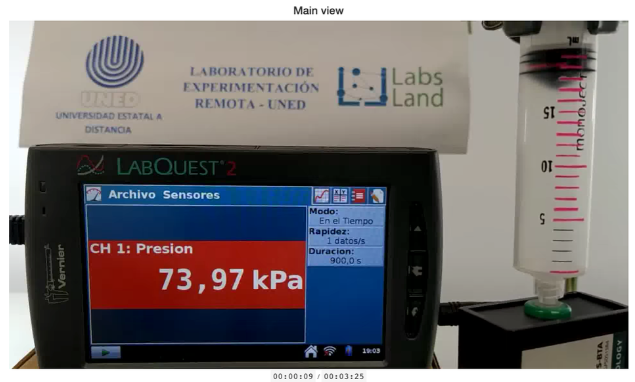
Camera perspective showing both the screen with information from the pressure sensor in kPa (kilopascals) and the syringe itself and its volume.